States of Matter: Solids, Liquids, and Gases
States of Matter: Solids, Liquids, and Gases
At the end of this lesson, you are expected to:
Describe the key properties of liquids, specifically that they have a definite volume but take the shape of their container.
Explain the arrangement and motion of particles within a liquid using simple terms and diagrams.
Compare and contrast liquids with solids and gases based on particle behavior.
Gather around, future scientists! I have here a few containers: a tall, skinny glass; a short, wide bowl; and a regular cup. Inside my special "mystery bottle," I have a liquid – let's call it "Wonder Water."
Now, watch closely! I'm going to pour the Wonder Water from the bottle into the tall, skinny glass. What happens to the water? Does it stay in a little ball? No, it spreads out, right? What shape does it take? It takes the shape of the glass!
What if I pour it into the short, wide bowl? What shape will it take now? Yes, it will spread out again and take the shape of the bowl!
But here's a question: If I pour all the Wonder Water from the bottle into the bowl, will the amount of water change? Will it suddenly become more or less water? Think about it! Today, we're going to explore this amazing state of matter called liquids and uncover their secrets!
Welcome, explorers! We've already learned that matter exists in different states, like solids (think of a sturdy block of wood) and gases (like the air we breathe). Today, we're diving into the fascinating world of liquids!
Liquids are everywhere around us. Water, juice, milk, cooking oil, even the blood flowing in your veins – these are all examples of liquids. They seem simple, but they have unique properties that make them special.
Property 1: Liquids Have a Definite Volume
Remember our "Wonder Water"? When I poured it from the bottle to the glass, and then to the bowl, did the amount of water change? No! If I started with 100 milliliters (mL) of Wonder Water, I still had 100 mL, no matter which container I poured it into.
This is a super important property of liquids: Liquids have a definite volume. This means that a liquid occupies a specific amount of space, and that amount doesn't change just because you put it in a different container.
Think about it: If you have a glass with 200 mL of juice, and you pour it into a larger pitcher, you still have 200 mL of juice. The juice doesn't magically become 500 mL just because the pitcher is bigger.
Contrast with Gases: Gases are different! If you release air from a balloon, it spreads out to fill the entire room. A gas does not have a definite volume; it expands to fill whatever container it's in.
Contrast with Solids: Solids also have a definite volume. A rock takes up the same amount of space whether it's on the ground or in your pocket.
Property 2: Liquids Take the Shape of Their Container
Now, let's go back to the shape. When we poured the Wonder Water into the tall glass, it became tall and skinny. When we poured it into the wide bowl, it became short and wide.
This is the second key property of liquids: Liquids take the shape of their container. Unlike solids, which keep their own shape, liquids flow and spread out to fill the bottom of whatever container they are placed in.
Real-World Example 1: Making Juice: Imagine you have a carton of orange juice (a liquid). If you pour it into a tall, narrow glass, the juice fills the glass and looks tall and narrow. If you pour the same juice into a wide, flat plate, the juice spreads out thinly across the plate. The juice itself doesn't change its amount (volume), but its shape changes to match the container.
Real-World Example 2: Cooking: When you cook soup, you pour it from a pot into a bowl. The soup, being a liquid, conforms to the shape of the bowl. If you were to pour a solid block of cheese into the same bowl, it would keep its block shape (unless it melts!).
Why Do Liquids Behave This Way? The Particle Model!
To understand why liquids have these properties, we need to look at the tiny particles (atoms or molecules) that make them up. Remember the particle model we talked about? It's super useful here!
In liquids, the particles are:
Close Together, but Not Fixed: Unlike solids, where particles are packed very tightly in a fixed pattern (like soldiers standing in formation), the particles in a liquid are still quite close to each other. However, they are not locked into place. Think of them like a crowd of people in a room – they are close, but they can move around.
Constantly Moving and Sliding: The particles in a liquid have enough energy to move past one another. They slide, glide, and tumble around. This constant motion is why liquids can flow. Imagine people in that crowd shuffling their feet and moving past each other.
Weakly Attracted to Each Other: The particles in a liquid are attracted to each other, but not as strongly as in solids. This weaker attraction allows them to move past each other but keeps them from flying apart completely (which is what happens in gases).
Visualizing Liquid Particles:
Let's draw it!
Solid: Imagine a box filled with neatly arranged marbles, all touching each other in rows and columns. They can only vibrate in place.
[O O O]
[O O O]
[O O O]Liquid: Now imagine the same box, but the marbles are jumbled up. They are still close together, touching or almost touching, but they are not in neat rows. They can roll and slide past each other.
[ O O O]
[ O O O ]
[ O O O]Gas: Finally, imagine the box with only a few marbles, spread far apart, bouncing off the walls and each other.
[O O]
[ O ]So, because the particles in a liquid are close but can slide past each other, the liquid can change its shape to fit its container. And because the particles stay relatively close together and are still attracted to each other, the liquid maintains a definite volume. They don't spread out infinitely like gases do.
Let's Summarize the Particle Behavior:
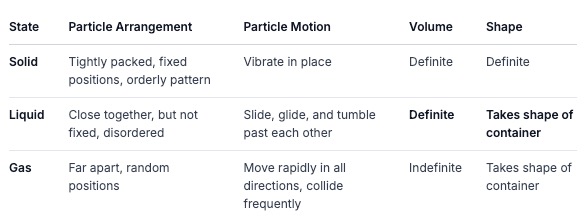
More Real-World Examples:
Washing Dishes: When you wash dishes, the water flows around the plates and cups, cleaning them. The water takes the shape of the spaces between the dishes and the sink.
Filling a Water Bottle: You can pour water from a large jug into a small water bottle. The water's volume stays the same, but it fills the bottle's shape.
Rain: Raindrops are liquids. They fall from clouds and take the shape of the raindrops themselves (due to surface tension, a related concept!), but when they land, they spread out on the ground or flow into puddles, taking the shape of the surface.
Changes of State Involving Liquids:
Liquids are often in the middle of a change of state!
Melting (Solid to Liquid): When a solid, like ice, gains enough heat energy, its particles start vibrating more and more until they break free from their fixed positions and begin to slide past each other. Ice melts into water.
Freezing (Liquid to Solid): When a liquid, like water, loses heat energy, its particles slow down. The attractions between them become strong enough to lock them into fixed positions, forming a solid (ice).
Evaporation (Liquid to Gas): When a liquid gains enough heat energy, some particles at the surface gain enough energy to break free from the attractions of other particles and escape into the air as a gas (water vapor). This is why puddles disappear on a sunny day.
Condensation (Gas to Liquid): When a gas, like water vapor in the air, loses heat energy, the particles slow down. The attractions between them become strong enough to pull them closer together, forming a liquid. This is how clouds form or how water droplets appear on the outside of a cold glass.
Read each statement below. Decide if it correctly describes a property of liquids. Write "Correct" or "Incorrect" next to each statement.
Liquids have a definite shape. _______
Liquids take the shape of their container. _______
Liquids have a definite volume. _______
The particles in a liquid are completely still. _______
The particles in a liquid are close together but can slide past each other. _______
Gases have a definite volume, but liquids do not. _______
(Answers: 1. Incorrect, 2. Correct, 3. Correct, 4. Incorrect, 5. Correct, 6. Incorrect)
What you need:
A small amount of water (or juice, or any safe liquid)
Several different containers: a tall glass, a wide bowl, a small cup, maybe even a plastic bag or a bottle.
A flat surface (like a table or tray) to work on.
Instructions:
Place about 50 mL (roughly 3-4 tablespoons) of liquid into one of the containers. Note its shape and volume (just estimate the amount).
Carefully pour the liquid into a different container.
Observe:
What happened to the shape of the liquid?
Did the amount (volume) of liquid seem to change?
Repeat this process, pouring the liquid into at least 3 different containers.
On a piece of paper, draw simple sketches of the liquid in each container. Label each sketch with the container's shape and write down your observations about the volume.
Be ready to share your observations and explain how they show the properties of liquids!
Look at the items below. Decide if each item is typically a liquid in its normal state. Write "Liquid" or "Not Liquid". Then, for the liquids, briefly describe one property they show.
Milk: _______ Property: _______
Rock: _______ Property: _______
Steam: _______ Property: _______
Cooking Oil: _______ Property: _______
Ice Cube: _______ Property: _______
Air: _______ Property: _______
(Example Answers: 1. Milk: Liquid, Property: Takes the shape of the glass it's poured into. 2. Rock: Not Liquid. 3. Steam: Not Liquid (it's a gas). 4. Cooking Oil: Liquid, Property: Has a definite volume. 5. Ice Cube: Not Liquid (it's a solid). 6. Air: Not Liquid (it's a gas).)
Liquids are essential for life and technology:
Drinking Water: We need liquids like water to survive. Our bodies are mostly made of water, and it helps transport nutrients and remove waste.
Transportation: Fuels like gasoline and diesel are liquids that power our cars, buses, and planes.
Cooking: Many cooking processes involve liquids, from boiling water for pasta to using oil for frying.
Industry: Liquids are used in countless industrial processes, like cooling machines, lubricating parts, and as ingredients in manufacturing products like paint and medicine.
Weather: Rain, rivers, lakes, and oceans are all bodies of liquid water that shape our planet and support ecosystems.
Understanding the properties of liquids helps us use them effectively and safely in our daily lives.
Today, we explored liquids! We discovered their two main properties:
Definite Volume: Liquids occupy a specific amount of space that doesn't change easily.
Takes the Shape of its Container: Liquids flow and adapt to the shape of whatever they are in.
We learned that these properties are explained by the particle model: liquid particles are close together but can slide and move past each other, unlike the fixed particles in solids or the widely spread particles in gases. This sliding motion allows liquids to flow and change shape, while their closeness keeps their volume definite.
Imagine you are explaining liquids to a younger child.
Choose one property of liquids (definite volume OR takes the shape of its container).
Think of a simple, fun way to demonstrate this property using things you can find at home (like water, different cups, maybe a spoon or a toy).
Describe your demonstration in a few sentences, as if you were explaining it to the child. What would you say and do?
For example, if you choose "takes the shape of its container," you might say: "Look! I have water here. See how it's in this round bowl? Now watch! I'm pouring it into this tall glass. Poof! It changed its shape to be tall like the glass! It's like magic water that can be any shape!"
No Comments Yet