States of Matter: Solids, Liquids, and Gases
States of Matter: Solids, Liquids, and Gases
At the end of this lesson, you are expected to:
Differentiate between solids, liquids, and gases based on the arrangement, spacing, and motion of their particles.
Describe the Particle Model of Matter as “All matter is made up of tiny particles with each pure substance having its own kind of particles.”
Describe that particles are constantly in motion, have spaces between them, attract each other, and move faster as the temperature increases (or with the addition of heat).
Use diagrams and illustrations to describe the arrangement, spacing, and relative motion of the particles in each of the three states (phases) of matter.
Explain the changes of state in terms of particle arrangement and energy changes: solid → liquid → vapor, and vapor → liquid → solid.
Look around you! What do you see? You probably see a desk, a chair, maybe a glass of water, and the air you breathe. All of these things are made of matter. But do they all seem the same?
Think about your desk. It keeps its shape, right? If you pick it up and move it, it stays a desk. Now think about the water in your glass. If you pour it into a different container, it takes the shape of that new container. And what about the air? You can’t even see it, but you know it’s there because you can feel it when the wind blows, and your lungs need it to breathe. It fills up whatever space it’s in.
These are all examples of matter, but they exist in different states or phases. Today, we’re going to explore these different states – solid, liquid, and gas – and understand what makes them unique by looking at the tiny particles that make them up!
Welcome, future scientists! Get ready to dive into the fascinating world of matter and its different states. Everything you see, touch, and even breathe is made of matter, and matter exists in different forms called states. The three most common states of matter are solid, liquid, and gas.
We're going to use a special idea called the Particle Model of Matter to understand why these states are so different. This model is super important because it helps us explain things we can't easily see – like the tiny building blocks of everything around us!
The Particle Model of Matter: The Tiny Building Blocks
Imagine that everything – your chair, the water, the air – is made up of incredibly tiny pieces called particles. Think of them like super-miniature LEGO bricks that build up everything in the universe.
Here are the key ideas of the Particle Model of Matter:
All matter is made of tiny particles: This is the most important rule! Whether it's a rock or a cloud, it's all made of these little particles.
Each pure substance has its own kind of particles: Just like there are different colors and shapes of LEGO bricks, there are different types of particles for different substances. For example, the particles that make up water are different from the particles that make up iron.
Particles are constantly in motion: These tiny particles aren't just sitting still. They are always moving! Think of them like busy little bees buzzing around.
Particles have spaces between them: There are tiny gaps or spaces between these moving particles.
Particles attract each other: There are forces that pull these particles together, like a gentle hug.
Particles move faster as the temperature increases (or with the addition of heat): When you add heat, it's like giving the particles more energy. They get more excited and move around faster and more wildly!
Understanding the States of Matter Through the Particle Model
Now, let's see how these particle ideas explain the differences between solids, liquids, and gases. We'll look at how the particles are arranged, how much space is between them, and how they move in each state.
1. Solids: The Organized and Tightly Packed
Think about a block of ice, a stone, or your textbook. These are all solids.
Particle Arrangement: In a solid, the particles are arranged in a very organized and fixed pattern, often like soldiers standing in neat rows or a tightly packed crystal. They have a specific shape.
Spacing: The particles in a solid are packed very closely together. There are very tiny spaces between them.
Motion: Because they are packed so tightly and attracted to each other strongly, the particles in a solid can't move around freely. They can only vibrate or shake in their fixed positions. Imagine them wiggling in place.
Energy: Solids have the least amount of energy compared to liquids and gases.
Diagram of Particles in a Solid:
* * *
* * * * *
* * *
* * * * *
* * *(Imagine these '' are particles, arranged neatly and close together)*
Example: A wooden table. It has a definite shape and volume. You can't easily squeeze it or change its shape without breaking it. The particles in the wood are held tightly in place, vibrating but not moving past each other.
2. Liquids: The Flowing and Close
Think about water, juice, or cooking oil. These are liquids.
Particle Arrangement: In a liquid, the particles are still close together, but they are not arranged in a fixed pattern. They can slide past each other. This is why liquids can flow and take the shape of their container.
Spacing: The particles in a liquid are still quite close, but there are slightly larger spaces between them compared to solids.
Motion: The particles in a liquid have more energy than in solids. They can move around, slide past each other, and roll over one another. This allows liquids to flow.
Energy: Liquids have more energy than solids but less energy than gases.
Diagram of Particles in a Liquid:
* * * *
* * *
* * * *
* * * *
* * *(Imagine these '' are particles, close but jumbled, able to move around each other)*
Example: Milk in a glass. The milk takes the shape of the glass. If you pour it into a bowl, it will take the shape of the bowl. The particles in the milk are close but can move and slide past each other, allowing the milk to flow.
3. Gases: The Free and Far Apart
Think about the air we breathe, steam from a kettle, or helium in a balloon. These are gases.
Particle Arrangement: In a gas, the particles are arranged randomly and are very far apart from each other. They move in all directions.
Spacing: There are large spaces between the particles in a gas.
Motion: Gas particles have a lot of energy! They move rapidly, randomly, and in straight lines until they bump into another particle or the walls of their container. They spread out to fill any available space.
Energy: Gases have the most energy among the three states.
Diagram of Particles in a Gas:
* *
*
* *
*
*(Imagine these '' are particles, very spread out and moving in random directions)*
Example: Air inside a balloon. The air fills the entire balloon because the gas particles are moving rapidly and spread out to occupy all the space. If you pop the balloon, the air rushes out and spreads into the room.
Comparing the States: A Quick Summary Chart
Let's put it all together in a chart to easily see the differences:
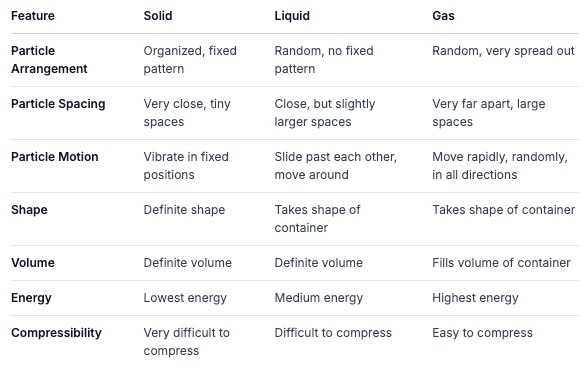
Changes of State: When Particles Get Excited (or Calm Down!)
What happens when you heat ice? It melts into water! And when you heat water enough, it turns into steam (water vapor). These are called changes of state. The Particle Model helps us understand these changes.
Solid to Liquid (Melting): When you add heat to a solid, its particles gain energy and start vibrating more vigorously. Eventually, they gain enough energy to break free from their fixed positions and start sliding past each other. This is melting, and the solid turns into a liquid.
Example: Ice melting into water. The particles in ice vibrate, and when heated, they move more and start flowing as liquid water.
Liquid to Gas (Evaporation/Boiling): When you add more heat to a liquid, its particles gain even more energy. They start moving faster and faster, and some particles at the surface gain enough energy to escape into the air as gas. If you heat the liquid enough, all the particles gain enough energy to spread far apart and move rapidly. This is evaporation or boiling, and the liquid turns into a gas.
Example: Water boiling in a kettle. The heat makes the water particles move so fast that they escape as steam (water vapor).
Gas to Liquid (Condensation): When a gas cools down, its particles lose energy. They slow down, and the forces of attraction between them start to pull them closer together. They start to clump together and slide past each other, forming a liquid.
Example: When you have a cold glass of juice on a hot day, water vapor from the air cools down when it touches the cold glass. The gas particles slow down, come together, and form tiny liquid water droplets on the outside of the glass. This is condensation.
Liquid to Solid (Freezing): When a liquid cools down, its particles lose energy and slow down. The forces of attraction between them become strong enough to lock them into fixed positions, where they can only vibrate. This is freezing, and the liquid turns into a solid.
Example: Water turning into ice in a freezer. The water particles slow down and arrange themselves into the fixed, vibrating pattern of ice.
Real-World Examples of States of Matter and Changes:
Cooking: When you cook an egg, the heat causes the proteins in the egg white to change from a liquid-like state to a solid state. This is a chemical change, but it shows how heat affects the structure of matter. When you boil water for your sinigang, you see the liquid water turn into steam (gas) as it boils.
Weather: Clouds are made of tiny water droplets or ice crystals (liquid or solid) floating in the air (gas). When these droplets or crystals get heavy enough, they fall as rain, snow, or hail. This involves changes of state from gas to liquid or solid.
Making Ice Cream: In the Philippines, we love ice cream! To make it, we often use a mixture of ice and salt. The salt lowers the freezing point of water, making the ice colder. This extreme cold causes the liquid ice cream mixture to freeze into a solid.
Why is this important? Understanding these states and how they change helps us in many ways, from cooking and understanding the weather to designing new materials and technologies.
Guided Practice: State Sorting
Let's practice identifying the states of matter! I will give you a list of items. For each item, decide if it is a solid, liquid, or gas. Think about its shape, whether it flows, and if it fills its container.
A rock
The air in your classroom
Juice in a pitcher
A wooden chair
Steam from a hot halo-halo
A bottle of soda
A metal spoon
The wind
A puddle of water
A diamond ring
(Think about each one and decide its state!)
Interactive Activity: Particle Motion Simulation (Imagine This!)
Let's imagine we are the particles!
Solid: Stand close together with your neighbors. Now, just wiggle in place. You can't move past each other, but you're vibrating!
Liquid: Now, stay close, but let go of your neighbors' hands. You can still touch each other, but now you can slide and move around each other. Try to flow around the space.
Gas: Spread out as much as you can! Move quickly in straight lines until you bump into a wall or another person. Then change direction and keep moving. Try to fill the whole room!
Now, imagine someone turns up the "heat" (energy). What happens to your movement in each state? (You'd move faster and more wildly!) What happens if the "heat" is turned down? (You'd slow down.)
Independent Practice: Draw the Particles!
On a piece of paper, draw three boxes. Label the first box "Solid," the second "Liquid," and the third "Gas."
Inside each box, draw circles or dots to represent the particles in that state. Make sure your drawings show:
The arrangement of particles (organized vs. random).
The spacing between particles (close vs. far apart).
The motion of particles (vibrating vs. sliding vs. moving freely).
You can use the diagrams from the lesson as a guide. Be creative!
Think about your day in the Philippines.
Morning: You drink water (liquid) or maybe juice (liquid). The air you breathe is a gas. The table you eat on is a solid.
School: Your notebook and pencil are solids. The chalk used by your teacher is a solid. The air in the classroom is a gas.
Afternoon: You might eat sorbetes (ice cream - solid) or drink buko juice (liquid).
Evening: When your family cooks dinner, you see water boiling (liquid to gas) and maybe rice cooking. The stove might be hot, transferring heat energy.
Even the clothes you wear are solids! Everything we interact with is in one of these states of matter. Understanding these states helps us understand how the world works, from cooking our favorite Filipino dishes to predicting the weather.
Matter is made of tiny particles.
These particles are always moving, have spaces between them, and attract each other.
Adding heat makes particles move faster.
In solids, particles are close, organized, and vibrate. Solids have a definite shape and volume.
In liquids, particles are close but can slide past each other. Liquids take the shape of their container but have a definite volume.
In gases, particles are far apart and move rapidly in all directions. Gases fill the entire volume of their container.
Changes of state (like melting, freezing, evaporation, condensation) happen when particles gain or lose energy, changing their arrangement and motion.
Now it's your turn to be a detective of matter!
Observe: Look around your home or classroom. Choose three different objects.
Identify: For each object, identify its state of matter (solid, liquid, or gas).
Explain: Explain why you think it is that state, using the ideas about particle arrangement, spacing, and motion. For example, "My water bottle is a liquid because the water inside takes the shape of the bottle, and if I tilt it, the water flows. This means the particles can slide past each other."
Bonus: Can you think of an example of a change of state happening in your home today? Describe it!
No Comments Yet