Changes of State: Melting, Freezing, Evaporation, and Condensation
Changes of State: Melting, Freezing, Evaporation, and Condensation
At the end of this lesson, you are expected to:
Explain evaporation as a process where liquid turns into gas due to increased particle energy.
Describe condensation as a process where gas turns into liquid due to decreased particle energy.
Relate evaporation and condensation to everyday phenomena, including their role in the water cycle.
Use diagrams to illustrate the particle arrangement and motion during evaporation and condensation.
Imagine you just finished playing outside after a light rain, and there's a small puddle on the ground. You come back a few hours later, and the puddle is gone! Where did the water go? Did it just vanish?
Think about this for a moment. What do you think happened to the water in the puddle? Write down your ideas.
Now, let's think about another situation. Have you ever noticed tiny water droplets forming on the outside of a cold glass of juice or water on a hot day? Where did that water come from? Did the glass leak?
These everyday mysteries are all about how water changes from one form to another. Today, we're going to become super scientists and uncover the secrets behind these changes!
Welcome, young scientists! Today, we're diving into the fascinating world of materials and how they can change. Specifically, we'll focus on how water, and many other liquids, can transform from a liquid into a gas, and then back again. These amazing changes are called evaporation and condensation.
Remember our research insights? We learned that all matter is made up of tiny particles. These particles are always moving, and their movement changes with temperature. This is the key to understanding evaporation and condensation!
Have you ever seen a puddle disappear on a sunny day? Or noticed your wet clothes drying on a clothesline? This is evaporation in action!
What is Evaporation?
Evaporation is the process where a liquid turns into a gas (also called vapor). Think of it as the liquid "escaping" into the air.
How Does it Happen? The Particle Model in Action!
Let's use the particle model to understand this. Remember, liquids are made of tiny particles that are close together but can move around and slide past each other. They are attracted to each other, but not as strongly as in solids.
Particles in Motion: The particles in a liquid are constantly moving. They bump into each other and have energy.
Gaining Energy: When the liquid is heated (like by the sun or a stove), its particles gain more energy. They start moving faster and faster!
Escaping the Surface: At the surface of the liquid, some particles might gain just enough energy to overcome the attraction from their neighbors. These energetic particles break free from the liquid and float away into the air as a gas (water vapor). This is evaporation!
Think of it like this: Imagine a group of friends holding hands in a circle. If they start jumping and moving around faster and faster, some friends on the edge might get so excited they let go and run off! The liquid particles are like those friends, and the ones that run off are now gas particles.
Factors Affecting Evaporation:
Several things can make evaporation happen faster:
Heat (Temperature): The hotter it is, the more energy the particles have, and the faster they evaporate. This is why puddles disappear faster on a warm day than on a cool day.
Surface Area: Evaporation happens at the surface of a liquid. If you spread a liquid out over a larger area, more particles are exposed to the air, and it will evaporate faster. Think about spreading butter on toast versus having a blob of butter – the spread butter melts (or evaporates, if it were liquid) faster!
Air Movement (Wind): Wind blows away the vapor particles that have just escaped the liquid. This allows more liquid particles to escape. It's like clearing away the "crowd" at the surface so more can leave. This is why clothes dry faster on a windy day.
Type of Liquid: Some liquids evaporate faster than others. For example, rubbing alcohol evaporates much faster than water because its particles are less attracted to each other.
Real-World Example 1: Drying Clothes
When you hang your wet laundry outside, the water in the clothes evaporates. The sun's heat gives the water particles energy. If it's windy, the wind carries the water vapor away, allowing more water to evaporate. That's why your clothes get dry!
Real-World Example 2: Sweating
When you exercise or it's hot, your body sweats. Sweat is mostly water. As the sweat evaporates from your skin, it takes heat away from your body, which helps cool you down. This is a very important way your body regulates its temperature!
Diagram of Evaporation:
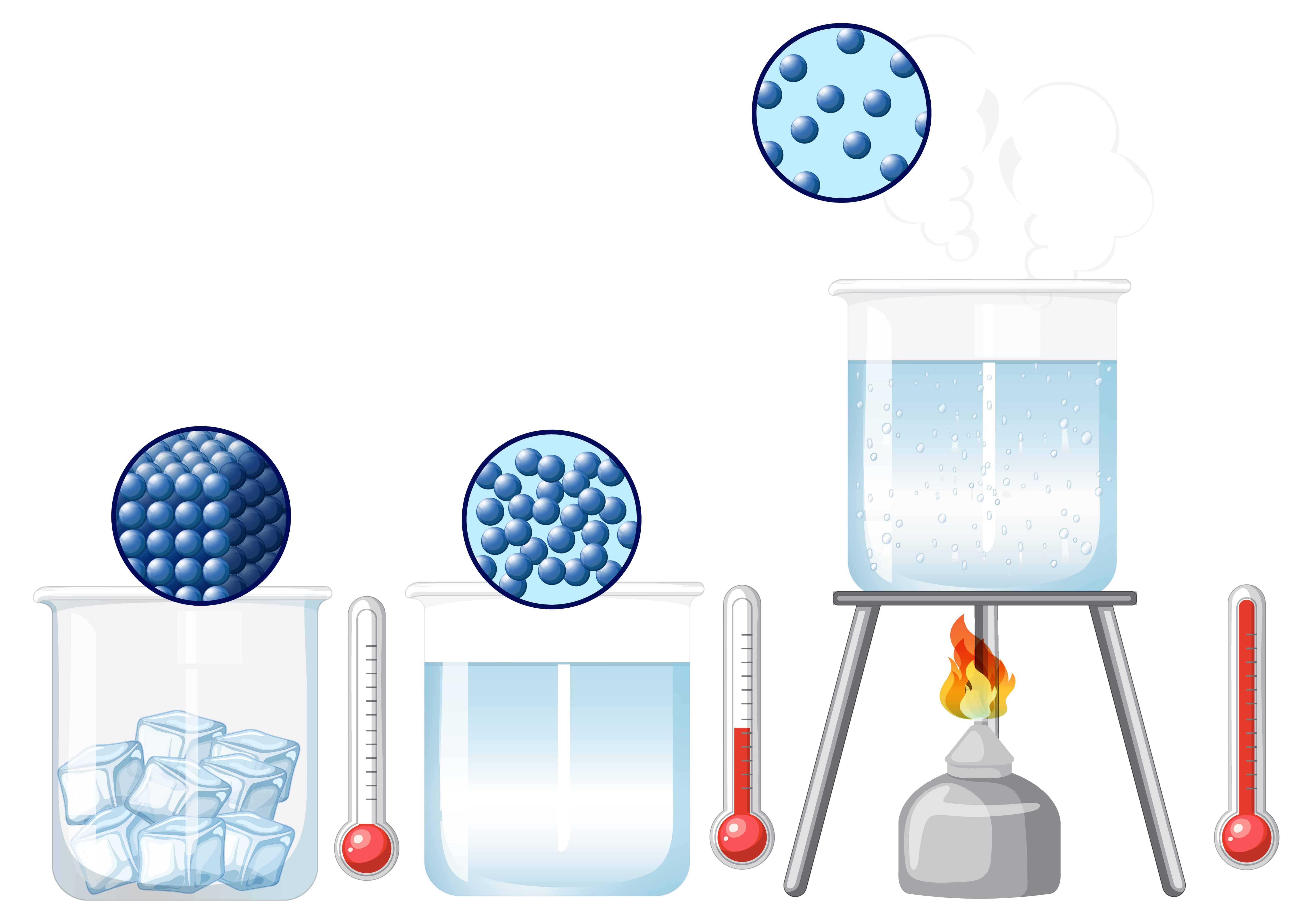
Liquid Water: Particles are close but can move around.
Evaporation: Heat energy makes some particles move faster. The fastest ones escape the surface as gas (vapor)
Arrangement: Particles spread out.
Spacing: Particles are far apart.
Motion: Particles move rapidly and randomly in all directions.
Now, let's think about the opposite process: condensation. Remember those water droplets on the outside of a cold glass? That's condensation!
What is Condensation?
Condensation is the process where a gas (vapor) turns back into a liquid. It's the reverse of evaporation.
How Does it Happen? The Particle Model Strikes Back!
Condensation happens when gas particles lose energy.
Gas Particles: Remember the particles that escaped during evaporation? They are now gas particles, moving fast and far apart.
Losing Energy: When these gas particles come into contact with a cold surface (like the outside of your cold glass), they lose energy. They slow down.
Coming Closer: As the gas particles slow down, the attractions between them start to pull them closer together. They clump up and form tiny liquid droplets.
Think of it like this: Imagine those friends who ran off earlier (the gas particles). If they suddenly get tired and cold, they might start to huddle together again, holding hands once more. They are turning back into a group that is closer together.
Real-World Example 1: Water Droplets on a Cold Drink
On a warm, humid day, the air is full of invisible water vapor. When this warm, moist air touches the cold surface of your glass, the water vapor particles lose energy, slow down, and condense into tiny liquid water droplets on the glass.
Real-World Example 2: Dew on the Grass
Early in the morning, especially after a cool night, you might see tiny water droplets on blades of grass and leaves. This is dew! During the night, the air cools down. The water vapor in the air loses energy and condenses onto the cooler surfaces of the plants.
Diagram of Condensation:
Imagine water vapor in the air.
Water Vapor (Gas): Particles are far apart and moving rapidly.
Condensation: Water vapor particles touch a cold surface, lose energy, slow down, and come together to form liquid droplets.
Arrangement: Particles get closer together.
Spacing: Particles become closer.
Motion: Particles slow down and move less randomly.
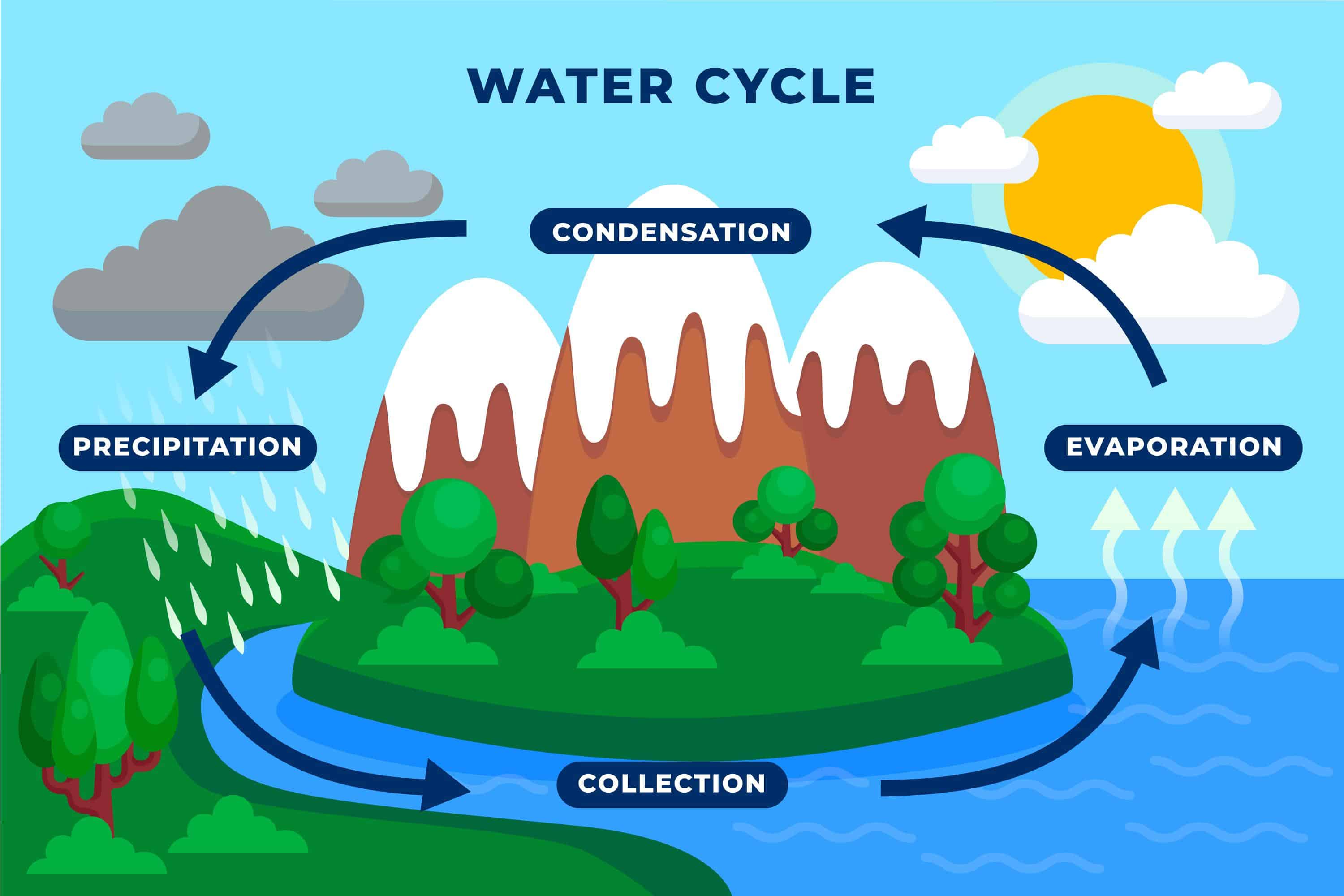
Evaporation and condensation are super important because they are key parts of the water cycle! The water cycle describes how water moves all around the Earth – in the oceans, in the air, and on land.
Here's how evaporation and condensation fit in:
Evaporation: The sun heats up water in oceans, lakes, and rivers. This water evaporates, turning into water vapor and rising into the atmosphere. Plants also release water vapor through a process called transpiration, which is like plant sweating!
Condensation: As the water vapor rises higher, the air gets colder. The water vapor cools down, loses energy, and condenses into tiny water droplets or ice crystals. These tiny droplets and crystals gather together to form clouds.
Precipitation: When the water droplets or ice crystals in the clouds become too heavy, they fall back to Earth as rain, snow, sleet, or hail.
Collection: The water that falls back to Earth collects in oceans, lakes, rivers, or soaks into the ground. Then, the cycle starts all over again with evaporation!
So, the water you see in a puddle today might have been in a cloud last week, or in the ocean long ago! It's all thanks to evaporation and condensation.
Diagram of the Water Cycle:
Key Concepts Recap:
Evaporation: Liquid → Gas. Particles gain energy, move faster, spread out.
Condensation: Gas → Liquid. Particles lose energy, slow down, come closer.
Particle Model: Explains these changes based on particle motion, spacing, and energy.
Water Cycle: A continuous process involving evaporation, condensation, and precipitation.
Let's test your understanding! Read the following scenarios and decide if evaporation or condensation is the main process happening.
Scenario: Steam rising from a hot cup of champorado.
Answer: Evaporation (liquid chocolate and rice mixture turning into steam/vapor)
Scenario: Fog forming on a bathroom mirror after a hot shower.
Answer: Condensation (hot water vapor from the shower cooling and turning back into liquid droplets on the mirror)
Scenario: A wet shirt left on the clothesline on a sunny, breezy day.
Answer: Evaporation (water in the shirt turning into vapor and going into the air)
Scenario: Tiny water droplets forming on the outside of a bottle of iced tea.
Answer: Condensation (water vapor from the surrounding air cooling and turning into liquid on the cold bottle)
Scenario: A puddle drying up after the rain stops.
Answer: Evaporation (liquid water turning into water vapor)
Materials:
A clear plastic cup or jar
Water
A small plate or lid that fits over the cup
Ice cubes (optional, but helpful!)
A sunny spot or a lamp (for gentle heat)
Instructions:
Pour about an inch of water into the clear plastic cup.
Cover the top of the cup tightly with the plate or lid.
Place a few ice cubes on top of the plate/lid (this makes the "cold surface" for condensation).
Place the cup in a sunny spot or under a gentle lamp.
Observe what happens for about 15-30 minutes.
What to Look For:
Do you see any "fog" or tiny droplets forming on the inside walls of the cup? (This is like evaporation happening).
Do you see any water droplets forming on the underside of the plate/lid? (This is condensation!).
If the droplets on the lid get big enough, they might even "rain" back down into the cup!
Think about it: How does this model show evaporation and condensation? What does the water in the cup represent? What does the plate/lid represent? What does the ice do?
Instructions:
Draw two simple diagrams side-by-side.
Diagram 1: Show a liquid (like water) in a container. Draw arrows and labels to show how the particles are arranged and moving. Then, show what happens during evaporation. Draw the particles spreading out and moving faster, escaping the container as gas. Label everything clearly (Liquid, Gas/Vapor, Particles, Heat Energy, Escaping).
Diagram 2: Show gas particles (like water vapor) in the air near a cold surface (like the outside of a cold glass). Draw arrows and labels to show how the particles are arranged and moving. Then, show what happens during condensation. Draw the particles slowing down, getting closer, and forming liquid droplets on the cold surface. Label everything clearly (Gas/Vapor, Liquid, Cold Surface, Particles, Losing Energy, Forming Droplets).
Make sure your drawings clearly show the difference in particle arrangement and motion between the liquid and gas states, and how they change during evaporation and condensation.
The Philippines is an archipelago, surrounded by water! This makes the water cycle incredibly important for us.
Rainfall: Evaporation from the vast oceans surrounding the Philippines is a major source of the water vapor that forms clouds. These clouds bring the rain that waters our farms, fills our rivers, and provides drinking water for our communities.
Drying Goods: Filipinos often dry fish, rice, and other goods in the sun. This relies on evaporation to remove moisture and preserve the food.
Cooling: On hot days, the evaporation of sweat helps keep us cool. Even the simple act of drying laundry relies on this natural process.
Weather: Understanding condensation helps us understand fog, clouds, and even the formation of typhoons, which are a significant part of our weather.
The continuous movement of water through evaporation and condensation is essential for life in the Philippines and all over the world.
Let's summarize what we've discovered today:
Evaporation is when a liquid turns into a gas (vapor). This happens when liquid particles gain enough energy to break free and move far apart.
Condensation is when a gas (vapor) turns back into a liquid. This happens when gas particles lose energy, slow down, and move closer together due to attraction.
The particle model helps us visualize these changes: in liquids, particles are close but mobile; in gases, they are far apart and move rapidly; during condensation, they slow down and get closer.
Factors like heat, surface area, and wind affect how quickly evaporation occurs.
Evaporation and condensation are vital parts of the water cycle, which moves water all around our planet.
Now it's your turn to be a material scientist in your own home or community!
Observe and Record: Over the next day, pay close attention to examples of evaporation and condensation around you. Keep a small logbook or notebook. Write down at least three examples you observe (e.g., "Water spots on the bathroom mirror after a shower," "Clothes drying on the line," "Dew on the grass this morning"). For each example, briefly explain whether it's evaporation or condensation and why.
Explain to Someone: Try explaining evaporation and condensation to a family member or friend using the ideas and diagrams we discussed today. See if you can help them understand how water changes state!
Think About Solutions: Can you think of any solutions you use at home where evaporation or condensation might be important? (Hint: Think about cooking or cleaning!)
By observing the world around you and explaining these concepts, you're becoming a true scientist! Keep exploring the amazing Science of Materials!
No Comments Yet