Changes of State: Melting, Freezing, Evaporation, and Condensation
Changes of State: Melting, Freezing, Evaporation, and Condensation
At the end of this lesson, you are expected to:
Identify and explain the different changes of state (solid, liquid, gas) in everyday Philippine scenarios.
Describe how temperature influences the movement and energy of particles during changes of state.
Connect the concepts of melting, freezing, evaporation, condensation, and sublimation to familiar events and processes.
Imagine you are helping out in the kitchen. You see water in a kettle on the stove, ice cubes in a glass of juice, and steam rising from a pot of rice. What do you think is happening to the water in each of these situations?
Think about how the water looks and feels. Is it always the same? What makes it change? Jot down your initial ideas. We'll explore these mysteries together in this lesson!
Have you ever wondered why ice melts into water, or why water turns into steam when you boil it? It all comes down to something called the Particle Model of Matter. Remember from our previous lessons that everything around us, even the air we breathe, is made up of tiny, tiny pieces called particles. These particles are always moving, and how they move and how close they are to each other determines whether something is a solid, a liquid, or a gas.
Let's dive deeper into this amazing world of particles and how they change!
1. The Particle Model: The Tiny Building Blocks of Everything
Our first key idea is the Particle Model of Matter. It tells us two very important things:
All matter is made up of tiny particles. Think of them like super-small LEGO bricks that build everything you see and touch – your chair, your water bottle, even you!
Each pure substance has its own kind of particles. This means the particles that make up water are different from the particles that make up salt, or sugar, or the air. These unique particles give each substance its special properties.
2. Particles in Motion: The Dance of Matter
These tiny particles aren't just sitting still. They are always on the move!
Constant Motion: Particles are constantly jiggling, wiggling, and moving around.
Spaces Between Them: There are tiny gaps or spaces between these particles.
Attraction: Particles are attracted to each other, like tiny magnets. This attraction helps hold them together.
Temperature and Speed: The hotter something gets, the faster its particles move! Think about how excited you get when you have a lot of energy – particles are similar! When you add heat (energy), they start moving faster and faster.
3. The Three States of Matter: Solid, Liquid, and Gas
The way these particles are arranged, how much space is between them, and how fast they move determines if something is a solid, a liquid, or a gas.
Solids: Imagine soldiers standing in neat, straight rows, very close together. They can only wiggle in their spots. In solids, particles are packed tightly, arranged in a regular pattern, and they only vibrate in fixed positions. This is why solids have a definite shape and volume. Think of an ice cube – it keeps its shape!
Example: An ice cube is a solid. Its water particles are packed very closely together in a rigid structure. They vibrate but don't move past each other.
Liquids: Now, imagine those soldiers are a bit more relaxed. They are still close, but they can slide past each other. In liquids, particles are still close but not in a fixed pattern. They can move around and slide past one another. This is why liquids can flow and take the shape of their container, but they still have a definite volume. Think of water in a glass – it spreads out to fill the bottom.
Example: Water in a glass is a liquid. The water particles are close but can move and slide past each other, allowing the water to flow and take the shape of the glass.
Gases: Imagine those soldiers are now running all over the place, far apart from each other, bumping into walls and each other. In gases, particles are very far apart and move very quickly in all directions. They have no definite shape or volume and will spread out to fill any container they are in. Think of the steam from a boiling kettle – it spreads out into the air.
Example: Steam from a boiling kettle is a gas. The water particles have gained so much energy that they move very fast and are far apart, filling the space around them.
4. Changes of State: The Great Particle Transformation!
Now, let's talk about how matter changes from one state to another. This happens when we add or remove heat (energy).
Melting (Solid to Liquid): When you heat a solid, its particles gain energy and start vibrating faster. Eventually, they gain enough energy to break free from their fixed positions and start sliding past each other. This is melting!
Real-World Example (Philippines): Think about halo-halo! When you take the leche flan or ube jam out of the freezer, it's solid. But as you add ice and other ingredients, the halo-halo gets colder, and if you leave it out too long, the solid parts like the leche flan or ube jam will start to melt into a liquid. The particles in the leche flan are gaining enough energy from the surroundings to move more freely.
Freezing (Liquid to Solid): When you cool a liquid, its particles lose energy and slow down. As they slow down, the attraction between them becomes stronger, and they start to lock into fixed positions, forming a solid. This is freezing!
Real-World Example (Philippines): Making ice cubes for your iced tea or juice! You start with liquid water in the ice tray. When you put it in the freezer, the cold removes heat energy from the water particles. They slow down, get closer, and arrange themselves into the solid structure of ice.
Evaporation (Liquid to Gas): When you heat a liquid, its particles gain energy and move faster. Some particles at the surface of the liquid gain enough energy to escape into the air as a gas. This is evaporation. It happens all the time, even without boiling!
Real-World Example (Philippines): After a rain shower, you notice puddles on the ground disappearing. Where does the water go? The sun's heat provides energy to the water particles in the puddles. These particles gain enough energy to escape into the air as water vapor (a gas). This is evaporation. Another example is when your nanay hangs the laundry outside. The water in the wet clothes evaporates into the air, leaving the clothes dry.
Condensation (Gas to Liquid): When you cool a gas, its particles lose energy and slow down. As they slow down, the attraction between them becomes stronger, and they start to clump together, forming a liquid. This is condensation.
Real-World Example (Philippines): Have you ever noticed tiny water droplets forming on the outside of a cold glass of soda or juice on a hot day? That's condensation! The water vapor (gas) in the warm, humid Philippine air touches the cold glass. The water vapor particles lose energy, slow down, and turn back into tiny liquid water droplets on the glass. Another example is when you see fog or clouds. These are tiny water droplets or ice crystals formed when water vapor in the air cools down and condenses.
Sublimation (Solid to Gas directly): Sometimes, a solid can turn directly into a gas without becoming a liquid first. This is called sublimation.
Real-World Example (Philippines): While less common in everyday cooking, dry ice (solid carbon dioxide) is a great example. On a hot day in the Philippines, if you see dry ice, it doesn't melt into a liquid; it turns directly into carbon dioxide gas, creating that smoky effect.
Deposition (Gas to Solid directly): The opposite of sublimation is deposition, where a gas turns directly into a solid.
Real-World Example (Philippines): Frost forming on cold surfaces on a very cold night is an example of deposition. Water vapor in the air turns directly into ice crystals without becoming liquid water first.
5. Diagrams: Visualizing the Particle Dance
Scientists use diagrams to show how particles are arranged and move in different states and during changes of state. These diagrams are super helpful for understanding!
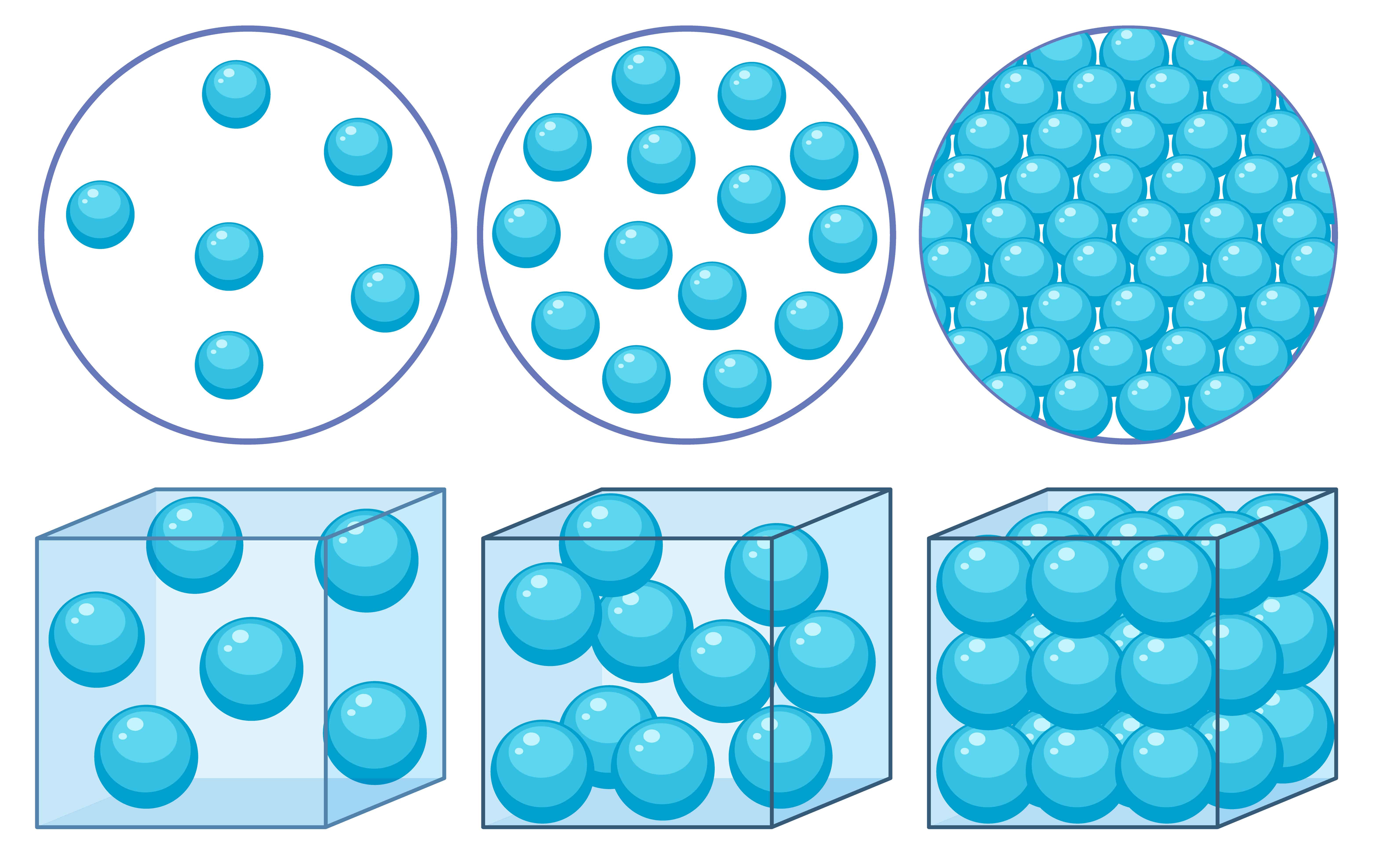
Solid: Particles are close, in a regular pattern, vibrating in place.
Liquid: Particles are close but randomly arranged, sliding past each other.
Gas: Particles are far apart, moving randomly and quickly.
Melting (Solid to Liquid): Add heat. Particles move faster, break free.
Freezing (Liquid to Solid): Remove heat. Particles slow down, lock into place.
Evaporation (Liquid to Gas): Add heat. Surface particles gain enough energy to escape.
Condensation (Gas to Liquid): Remove heat. Gas particles slow down, clump together.
These diagrams help us "see" what's happening at a level we can't normally observe!
Guided Practice: "What's Happening Here?"
Let's look at some common situations in the Philippines and identify the changes of state. I'll describe them, and you tell me what's happening with the particles!
Boiling Rice: Your lola is cooking rice. You see bubbles forming in the boiling water and steam rising.
What state is the water initially? (Liquid)
What happens when it boils? (It turns into steam, which is a gas.)
What change of state is this? (Evaporation or Boiling)
What is happening to the water particles as they turn into steam? (They are gaining energy, moving faster, and spreading far apart.)
Making Ice Cream: You're making homemade ice cream. You mix the ingredients and then put the mixture in a container surrounded by ice and salt.
What state is the ice cream mixture initially? (Liquid)
What happens after some time in the cold mixture? (It becomes solid.)
What change of state is this? (Freezing)
What is happening to the particles in the mixture as it freezes? (They are losing energy, slowing down, and arranging themselves into a solid structure.)
Dew on Grass: Early in the morning, you notice tiny water droplets on the blades of grass, even though it didn't rain.
What state is the water initially? (Water vapor in the air - a gas)
What happens to the water vapor when it touches the cool grass? (It turns into liquid water droplets.)
What change of state is this? (Condensation)
What is happening to the water vapor particles? (They are losing energy, slowing down, and coming together to form liquid droplets.)
Interactive Activity: "State Change Charades!"
Let's play a game! I'll give you a situation, and you act out the change of state.
Instructions:
Read the situation.
Think about the state of matter involved and how the particles are moving.
Act out the change of state using your body. You can be the particles!
Solid: Stand still, maybe wiggle slightly in place.
Liquid: Move around slowly, sliding past imaginary neighbors.
Gas: Move around quickly and randomly, spreading out.
Melting: Start as a solid, then slowly become more fluid like a liquid.
Freezing: Start as a liquid, then slow down and become rigid like a solid.
Evaporation: Start as a liquid, then move faster and spread out like a gas.
Condensation: Start as a gas, then slow down and come together like a liquid.
Situations:
An ice cube left on the table on a hot day. (Melting)
Water being poured into a glass. (Liquid state, no change)
Steam rising from a hot bowl of sinigang. (Evaporation/Boiling)
Water turning into ice in the freezer. (Freezing)
Fog forming in the mountains. (Condensation)
A block of butter melting in a hot pan. (Melting)
Independent Practice: "My Own Change of State Story"
Now it's your turn to be creative! Think of a situation in your daily life in the Philippines where a change of state happens. It could be related to food, weather, or anything else!
Instructions:
Choose a situation. (Examples: Making kakanin, drying clothes, a foggy morning, a cold drink sweating.)
Write a short story or description (at least 5 sentences) about your chosen situation.
In your story, clearly mention the substance that is changing state.
Explain what change of state is happening (melting, freezing, evaporation, condensation).
Describe what the particles are doing before and after the change, and how temperature is involved.
Example Story Idea: "It was a very hot afternoon in Manila. I had just taken a cold bottle of water from the refrigerator. As I held it, I noticed tiny water droplets forming on the outside of the bottle. The water vapor in the warm air was touching the cold bottle, losing energy, slowing down, and turning into liquid water. This process is called condensation!"
Understanding changes of state is super important because it helps us understand so many things around us, especially in the Philippines!
Cooking: From boiling water for sinigang to freezing sorbetes, changes of state are happening all the time in our kitchens. Knowing about them helps us cook better and understand how different foods are made.
Weather: The Philippines experiences a tropical climate with rain, humidity, and sometimes fog. Understanding condensation helps explain how clouds form and why we get rain. Evaporation is what dries our clothes and fills the air with moisture.
Industry: Many industries rely on changes of state. For example, power plants use the evaporation of water to create steam that turns turbines. Refrigerators use the process of evaporation and condensation to keep things cool.
Our Bodies: Even our bodies use changes of state! When we sweat, the evaporation of sweat from our skin helps cool us down.
By understanding these simple concepts, you can better understand the world around you, from the food you eat to the weather you experience!
Let's review what we've discovered:
Matter is made of tiny particles that are always moving.
The state of matter (solid, liquid, gas) depends on how these particles are arranged, how much space is between them, and how fast they move.
Temperature is key! Adding heat gives particles more energy and makes them move faster, leading to changes like melting and evaporation. Removing heat makes particles slow down, leading to freezing and condensation.
We see changes of state everywhere: ice melting, water boiling, puddles drying up, and dew forming on grass.
Now, put your knowledge to the test!
Observation Challenge: Over the next day, try to spot at least three different examples of changes of state happening around you. Write them down and explain what is happening using the terms you learned in this lesson (particles, energy, solid, liquid, gas, melting, freezing, evaporation, condensation).
Explain it to Someone: Imagine you need to explain to a younger sibling or a friend why a cold glass of water gets wet on the outside. Use your own words and maybe even draw a simple picture to show them what's happening with the particles.
Keep observing the world around you, and you'll see the amazing science of materials and changes of state everywhere!
No Comments Yet